Degree of Dissociation Formula
Three reasons for that. The amount of heat supplied to heat an object can be expressed as.

Calculate The Degree Of Dissociation Of Hi At 450 C If The Equilibrium Constant For The Diss Youtube
Optical transmission properties provide a means for distinguishing among various types of vitreous silica as the degree of transparency reflects material purity and the method of manufacture.
. Acidity is defined as a pH of less than 7. Phosphoric Acid is an acid-containing four atoms of oxygen one atom of phosphorus and three atoms of hydrogen. Q amount of heat supplied J Btu C heat capacity of system or object JK Btu o F dt temperature change.
This is derived from the molarity of protons hydrogen ions or H in the solution. Q C dt 1 where. In reality the answer will be slightly different.
A weak acid is only partially dissociated with. The degree to which a base dissociates can be represented as a percentage. The UV cutoff ranges from 155 to 175 nm for a 10 mm thick specimen and.
Phosphoric Acid is a weak acid with the chemical formula H 3 PO 4. Eliot in full Thomas Stearns Eliot born September 26 1888 St. Heat Capacity - C - is a characteristic of an object - the amount of heat required to change its temperature by one degree.
Similarly if a fraction of moles of solute associate to form one mole of an n-mer dimer trimer etc then. Central depression at very high levels of pCO2. Cerebral vasodilation increasing cerebral blood flow and intracranial pressure.
Relationship between Kp and Kc is given by K p K c RT Δn where K p and K c are the equilibrium constants for an ideal gaseous mixture. If the acidosis persists a decrease in red cell 23 DPG occurs which shifts the curve back to the left. This is case of strong acid titrated with strong base so we expect pH at equivalence point to be that of neutral solution - that is 700.
The pH scale which normally spans from 0 to 14 in water is used to determine the pH of an aqueous solution. Eliot exercised a strong influence on Anglo. Specific indicators are the UV cutoff and the presence or absence of bands at 245 nm and 273 um.
First sulfuric acid has pK a1 -3 very strong acid but second dissociation step has pK a2 20 so it is much weaker. For dissociation in the absence of association the van t Hoff factor is. Dissociation ionization OH-at equilibrium base initial 100 i If dissociation 100 the base is a strong base ii If dissociation is small the base is a weak base Please do not block ads on this website.
A pH of more than 7 is classified as basic. K p is the equilibrium constant used to measure equilibrium concentrations represented in atmospheric pressureWhen equilibrium concentrations are expressed in molarity K c is the equilibrium constant used. Examples of strong acids are hydrochloric acid perchloric acid nitric acid and sulfuric acid.
Heat Capacity has the units of energy per degree. Here we will study the pH value formula and how pH value is calculated in detail. A double replacement reaction will occur if a formation of a precipitate gas or water takes place.
Assuming 100 dissociation calculate the freezing point Tf and boiling point Tb of 327 m K3PO4aq. Acid strength is the tendency of an acid symbolised by the chemical formula to dissociate into a proton and an anion The dissociation of a strong acid in solution is effectively complete except in its most concentrated solutions. No ads no money for us no free stuff for you.
A pH of 7 is regarded as neutral. To find pH for a given molarity you need to know how to work with logarithmic equations and a pH formula. Acutely the acidosis will cause a right shift of the oxygen dissociation curve.
Colligative constants in degrees Celciusm Kf value of water. Louis Missouri USdied January 4 1965 London England American-English poet playwright literary critic and editor a leader of the Modernist movement in poetry in such works as The Waste Land 1922 and Four Quartets 1943. First week only 499.
The pH scale ranges from 0 to 14 under usual conditions and measures the acidity of an aqueous solution. Course Summary Chemistry 101. It is present in teeth and bones and helps in.
Assuming 100 dissociation calculate the freezing point Tf and boiling point Tb of 327 m K3PO4aq. General Chemistry has been evaluated and recommended for 3 semester hours and may be transferred to over 2000 colleges and universities. Select two compounds above and this calculator will predict whether or not the reaction will occur in waterThis is simply based on the solubility chart of inorganic compounds.
2 CH 3 COOH CH 3. Start your trial now. Orthophosphoric acid refers to phosphoric acid.
The degree of dissociation is the fraction of the original solute molecules that have dissociated. It is also known as phosphoricV acid or orthophosphoric acid. Potent stimulation of.
For the dimerisation of acetic acid in benzene.

Degree Of Dissociation Definition Examples Diagrams

Calculate The Percentage Degree Of Dissociation Of An Electrolyte Xy 2 Normal Molar M Youtube

Degree Of Association And Degree Of Dissociation Youtube
If The Degree Of Dissociation Is Given Then How To Take The Values At Equilibrium Q 2hi H2 I2 The Degree Of Dissociation Is A Calculate Expression For Equilibrium Constant Kc Of The

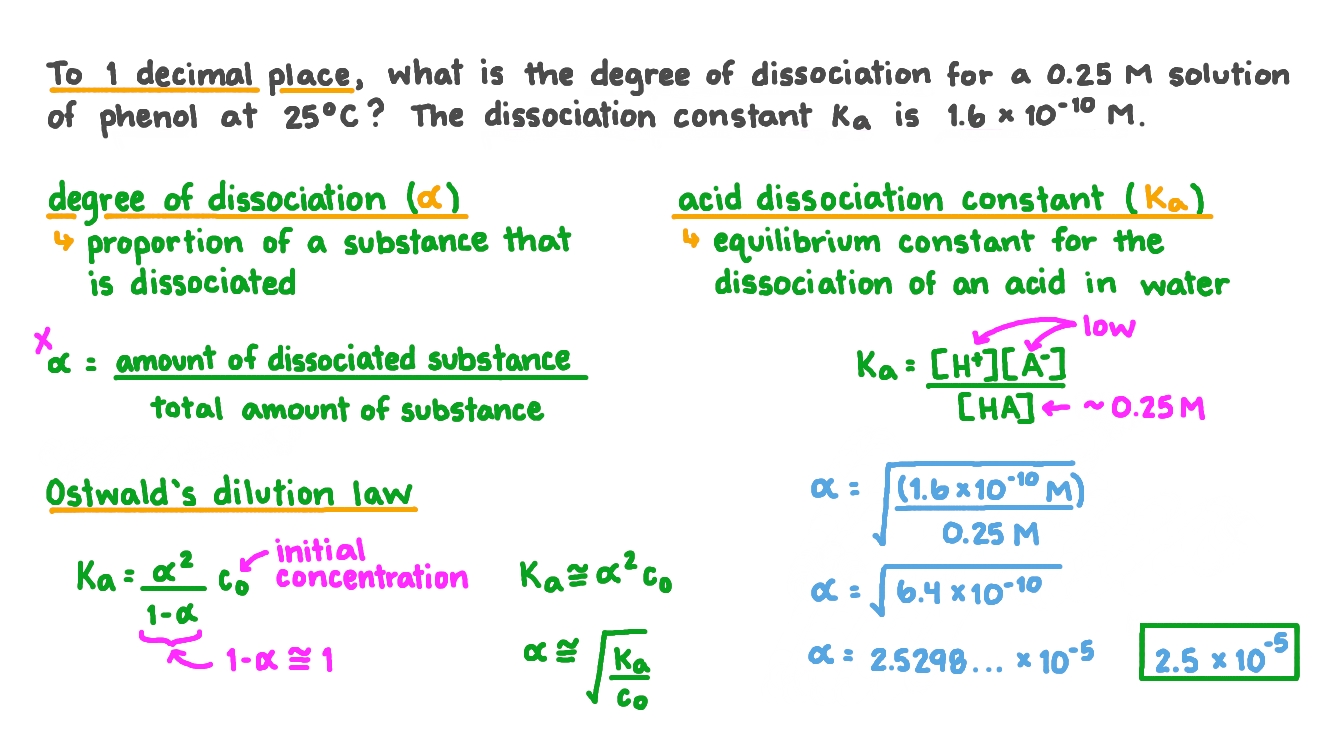
Question Video Calculating The Degree Of Dissociation Of A Solution Of Phenol Given The Acid Dissociation Constant Nagwa

Determine Degree Of Dissociation Of 0 05m Nh 3 At 25 C In A Solution Of Ph 11 Youtube
0 Response to "Degree of Dissociation Formula"
Post a Comment